What is the structure of XeF6 compound. The fluorines are not attached to the xenon with a covalent bond.
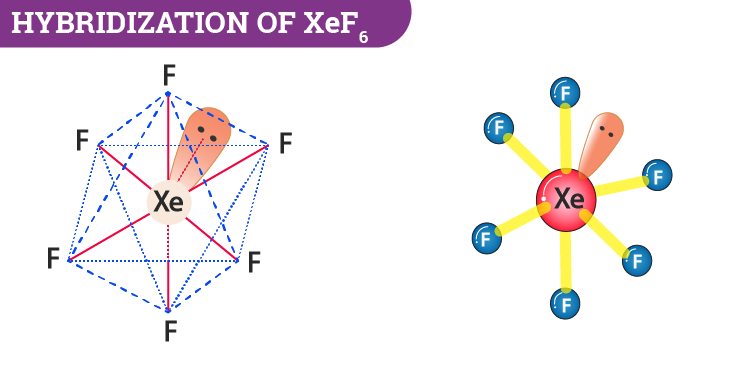
Hybridization Of Xef6 Hybridization Of The Xenon Atom In Xef6
How do you determine and draw the Lewis structure of XeF6.
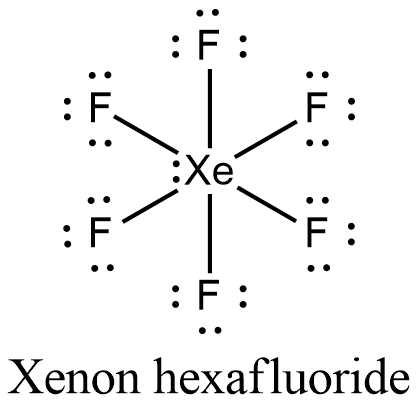
Lewis structure for xef6. A step-by-step explanation of how to draw the XeF6 Lewis Dot Structure Xenon hexafluorideFor the XeF6 structure use the periodic table to find the total n. The Lewis structure for XeF 6 requires you to place more than 8 valence electrons on Xe. Xenonhexafluorid ist eine chemische Verbindung aus der Gruppe der Edelgasverbindungen.
Oxidation number is 0 for atoms in an element. Xenon Xe can have more than 8 valence electrons in your Lewis structure. Lewis Structure for XeF2 - UM.
Die Strukturen der Xe-Fluoride können generell nach dem VSEPR-Konzept erklärt werden. Danach ist Xe F 2 linear drei freie Elektronenpaare Xe F 4 quadratisch-planar zwei freie Elektronenpaare gebaut. Draw the lewis structure to describe the shape and give the approximate bond angles of the following ions.
By signing up youll get thousands of step-by-step solutions to your homework. A step-by-step explanation of how to draw the BrF2- Lewis Dot StructureFor the BrF2- structure use the periodic table to find the total number of valence el. Xe is a noble gas and F is a halogen.
So Xe has 8 valence electrons and F has 7. Considering there are 6 Fs thats 6 x 7 42 valence electrons from Fs. Draw structure of following compound XeF4 XeF6XeO3XeOF2.
Drawing Lewis Structures Exceptions XeF6. Steve Jobs introduces iPhone in 2007 - Duration. Valence electronic structures can be visualized by drawing Lewis symbols for atoms and monatomic ions and Lewis structures for molecules and polyatomic ions.
Download Xef6 Lewis Structure Molecular Geometry PNG. XeF6 Lewis Structure In the XeF6 Lewis structure Xe is the least electronegative and goes at the center of the structure. 73 Lewis Symbols and Structures Chemistry opentextbcca.
Since Xe is the least electronegative of t. So in total we have 42 8 50 valence electrons. Most structuresespecially those containing second row.
The Lewis structure for XeF6 requires you to place more than 8 valence electrons on Xe. This doesnt compare well to molecular structures as are present in xenon compounds which feature distinct ceXeF_2n molecules. Lone pairs unpaired electrons and single double or triple bonds are used to indicate where the valence electrons are located around each atom in a Lewis structure.
Danach ist XeF 2 linear drei freie Elektronenpaare XeF 4 quadratisch planar gebaut. 12142011 101022 P XeF6 Lewis Structure - How to Draw the Lewis Structure for XeF6 - Duration. Draw The Lewis Dot Structure For Xef6 How to determine and draw the Lewis structure of XeF6 Quora XeF6 Lewis Structure How to Draw the Lewis Structure for Xef6 Structure XeF4 Lewis Structure How to Draw the Lewis Structure.
Xenon hexafluoride has sp3d3. XeF6 has seven pairs of electrons to distribute as far apart as possible according to VSEPR theory. The lewis structure for xef6 requires you to place more than 8 valence electrons on youll want to calculate the formal charges on each atom to make.
If you are looking for a different one maybe clarify it. Draw and explain the molecular structure of XeF6. Therefore XeF6 has a sp3d3 hybridisation and a Distorted Octahedral Structure.
So the distorted octahedral structure is the best geometrical choice. Endgroup Jan Dec 17 16 at 1747 1 begingroup Correct to a first approximation. It includes the general shape of the molecule as well as bond lengths bond angles torsional angles and any other geometrical parameters that determine the position of each atom.
XeF6 Struktur Xenonhexafluorid - Wikipedi. XeF6 Lewis Structure - How to Draw the Lewis Structure for. The structure of XeF6 will be This will be called as a Distorted Octahedral structure keeping in mind that the position of the lone pair of electrons is not taken into consideration.
They are simply attracted to it. Xe F 6 ein c XeF4 quadratischer Bau VSEPR sagt quadratisch planare Struktur aber abgeleitet von einer oktaedrischer Pseudostruktur AX4E2 -. Wayne Breslyn 24723 views.
The 3c4e model doesnt fully explain XeF6 though as it. So all these bonds will take up 12 valence electrons now that we know the lewis structure of sf6 we can now determine the atoms hybridization in the molecule. Xef6 Lewis Structure.
The extra pair of electrons is said to do what might be thought of as a. Es ist ein farbloser Feststoff. Xenon Xe can have more than 8 valence electrons in your Lewis structure.
In the XeF 6 Lewis structure Xe is the least electronegative and goes at the center of the structure. XeF6 Xe F 7 Xe F 8 2 Die Strukturen der Xe-Fluoride lassen sich gut nach dem VSEPR-Konzept erklären.